The Magic of Transforming Carbon Dioxide to Ethanol: An Analysis of Copper-Based Electrochemical Catalysts
Copper electrocatalysts have been shown in the past literature to selectively reduce carbon dioxide to hydrocarbons. However, due to the lack of systematic studies on time-scale resolved spectral features, the selectivity resulted from the surface atomic characteristics of the copper catalyst– could be copper metal or copper oxide, along the reaction path remains uncertain. This study introduces an X-ray absorption spectrum with a few seconds resolution scale to observe the chemical state evolution of catalysts in reactions. Under specific experimental conditions, it was observed that the average oxidation state consisted of the mixed oxidation states on the surface of copper material could be stably maintained on the electrode surface, thus steadily increasing the effective yield of ethanol. Combined with the first-principles simulations, the catalytic reaction pathway of CO2 conversion can be derived step-by-step and verified by experimental observations.
Since the Industrial Revolution, the use of energy by humans and society has increased dramatically. In order to meet the energy shortage required for the stable operation of national societies, fossil fuels such as oil, coal, and natural gas, which have long been stored in the earth's crust, are extracted through the enhancement of drilling technology and converted into electric, mechanical, or thermal energy for daily use through chemical combustion reactions. As a result, the emission of carbon dioxide (CO2) into the atmosphere seems to be a necessary evil for technological progress. The National Oceanic and Atmospheric Administration of the USA (NOAA) has been observing the rising trend of CO2 concentration in the atmosphere for a long time, and this concentration has reached record highs several times in recent years (Figure 1: over 400 ppm). The global greenhouse effect caused by high CO2 concentration is gradually affecting the living environment of human beings, such as the rising sea level and extreme climate events. In view of this, the effective recovery and conversion of CO2 gas emissions into secondary useable fuels in chemical technology has become an important issue for countries to complete the circular economy model.
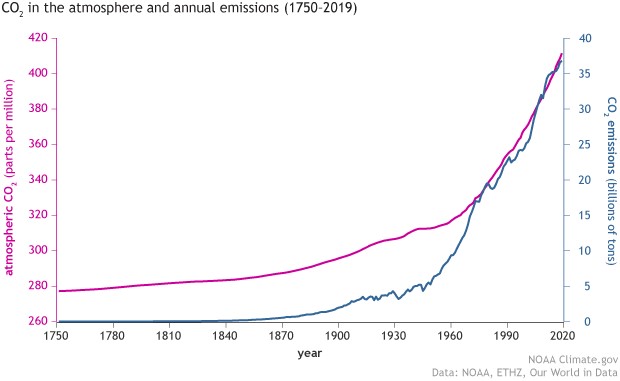
Figure 1: CO2 Atmospheric Concentration and Emissions (Source: www.noaa.gov)
CO2 is chemically the highest oxidation state of the carbon, meaning that it cannot react with oxygen for further combustion. If CO2 is to be converted into other fuels, it must be converted into other hydrocarbons under reaction conditions that provide electrons and hydrogen, which is chemically known as a reduction reaction. The possible reaction pathways are shown below:
CO2 + 2e– + 2H+ → H2O + CO (Carbon monoxide; one of the raw materials required for industrial processes)
CO2 + 2e– + 2H+ → HCOOH (Formic acid; antiseptic and antibacterial properties)
CO2 + 4e– + 4H+ → H2O + HCHO (Formaldehyde; raw material for industrial resins)
CO2 + 6e– + 6H+ → H2O + CH3OH (Methanol; can be used as cell fuel)
CO2 + 8e– + 8H+ → 2H2O + CH4 (Methane; one of the components of natural gas)
2CO2 + 12e– + 12H+ → 4H2O + C2H4 (Ethylene; an important raw material for polymer synthesis)
2CO2 + 12e– + 12H+ → 3H2O + C2H5OH (Ethanol or commonly known as alcohol; has fuel and medical uses)
From the reaction pathways listed above, it can be seen that there are many options for CO2 conversion. The higher the molecular weight of the product, the higher the technological threshold to be overcome. The effective conversion of CO2 to ethanol is a topic of interest for many scientists and has considerable potential economic value.
From the precious scientific literature, copper metal is a special electrode material that can convert CO2 into various hydrocarbons simultaneously in an electrochemical reaction environment (i.e., lack of single product selectivity). From a chemical process point of view, the more efficiently a single product can be synthesized, the higher the potential for technological applications to be derived from it. Copper oxide is a common product in the rusting of metallic copper, and through proper preparation, a special material can be produced that contains both cuprous (monovalent copper ions) and metallic copper on its surface. The previous scientific literature has reported that the presence of mixed oxidation states (i.e., divalent copper ions, cuprous ions, or metallic copper) on the surface of copper materials can effectively assist in the conversion of carbon dioxide to products containing carbon chemical bonds such as ethylene or ethanol.
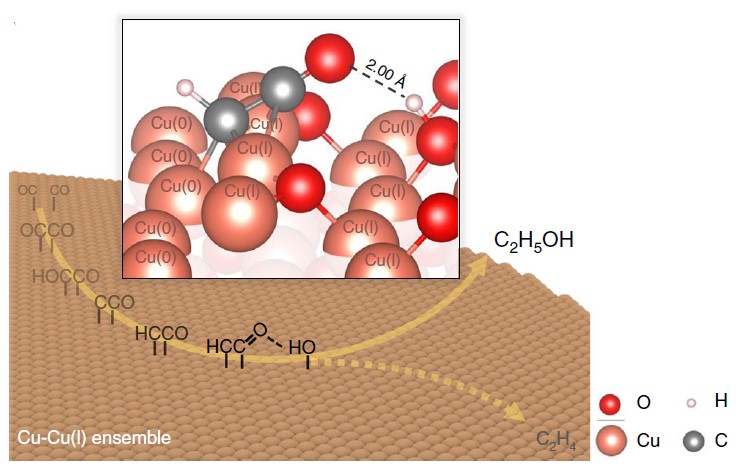
The formation of carbon-carbon bonds can be carried out by the heterogeneous bonding steps of CO dimerization. Through the unique intermittent pressure control technique and the monitoring of the in-situ spectral signal (X-ray absorption spectroscopy with a few seconds resolution scale), it was observed that the average oxidation state consisted of the mixed oxidation states on the surface of copper material can be steadily maintained at the electrode surface under specific experimental conditions, thus steadily increasing the effective production of ethanol (Figure 2).
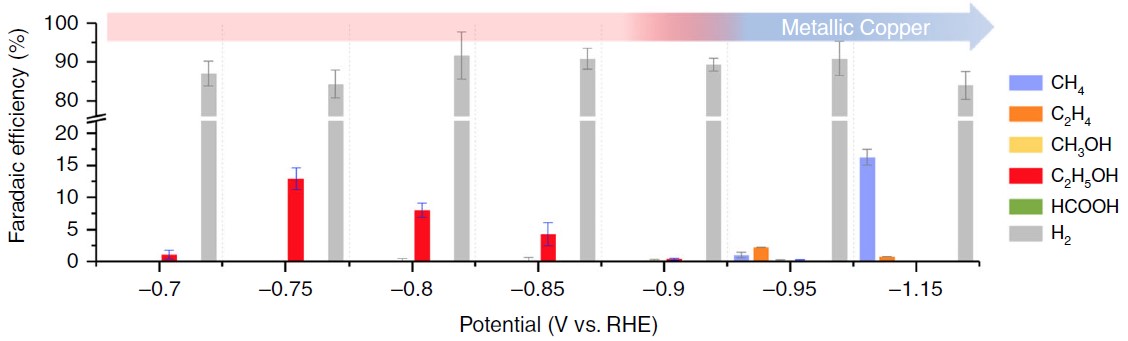
Fig. 2: Distribution of electrochemical catalytic products of CO2
During the study, the crystalline surface of Cu metal mixed with Cu2O (110) simulated by first-principles calculations could play a key role in elucidating the ethanol synthesis pathway (Figure 3). In the highly oxidized (as opposed to metallic) surface blocks rich in cuprous copper, the hydroxide functional group is an important factor in the protection of oxygen-containing intermediates in the CO2 reduction process through the attractive inter-dipole interactions.
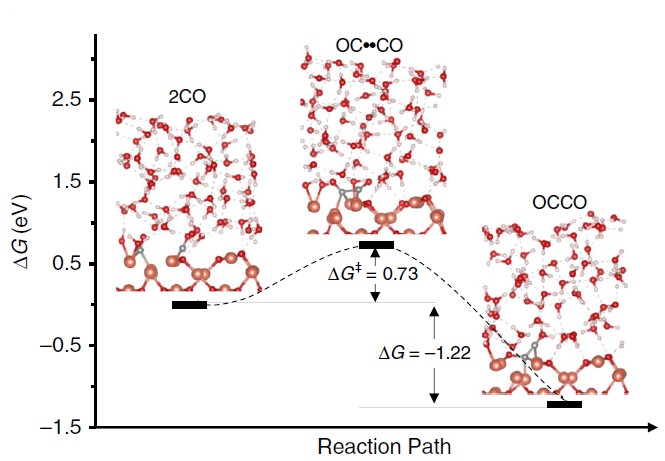
Figure 3: Predicted free energy profile of C-C bond formation (liquid-solid interface simulation at the full atomic scale)
This research was conducted by a team from National Taiwan Normal University (NTNU) to simulate and explore the crystalline surfaces of various copper surfaces, to identify highly reactive interfaces, and to rationalize the reaction mechanism. The theoretical prediction results were compared with the experimental observations – materials synthesis and in-situ spectroscopy analysis by Prof. Hao-Ming Chen's group in the Department of Chemistry, National Taiwan University. Through the collaboration of bilateral research teams, the synthesis and characterization of this novel Cu-based electrocatalysts were successfully carry out to explore the reaction mechanism for the conversion of CO2 to ethanol.
Reference:
'Operando Time-resolved X-ray Absorption Spectroscopy to Unravel the Chemical Nature: Chemical State-Trapping Strategy Enabling the Highly Selective CO2 Reduction', Sheng-Chih Lin, Chun-Chih Chang, Shih-Yun Chiu, Hsiao-Tien Pai, Tzu-Yu Liao, Chia-Shuo Hsu, Ming-Kang Tsai,* Hao Ming Chen,* Nat. Commun. 2020, 11, 3235.
https://www.nature.com/articles/s41467-020-17231-3
(This report is provided by Dr. Ming-Kang Tsai’s research team in the Department of Chemistry)
Ming-Kang Tsai Professor | Department of Chemistry
Prof. Ming-Kang Tsai received his PhD from University of Pittsburgh (US) in 2005. He worked as a postdoctoral researcher in the Pacific Northwest National Laboratory and Brookhaven National Laboratory of Department of Energy in the US from 2006-2010. He began his full-time faculty appointment at National Taiwan Normal University in 2010. His research interests focus on utilizing and developing high performance computing simulations, and combining programming and numerical analysis to design novel materials catalysts. He is also interested in applying big data analysis using machine learning approaches to advance computational predictions.

